Introduction: Patient-derived data can increase breadth of knowledge in rare cancers like Waldenström's Macroglobulinemia (WM), including patient-reported outcomes (PROs). WhiMSICAL (Waldenström's Macroglobulinemia Study Involving CArt-wheeL) is the only global registry capturing patient-derived data for hypothesis generation in WM. Rapidly adaptable, it has been amended to capture Coronavirus Disease 2019 (COVID-19) data.
Methods: An ethically-approved WM-specific extension to www.cart-wheel.org, an online rare cancer database for patient-derived data, was developed by clinician and patient investigators. Participants complete consent, and enter symptom, pathology, treatment and PRO (EORTC-QLQ-C30, Impact of Event Scale-6) data online. Recruitment strategies utilizing social media tools are driven by the International Waldenström's Macroglobulinemia Foundation investigators. A validation study compared patient-entered data with data-manager-entered data in the Australia & New Zealand Lymphoma & Related Diseases Registry (LaRDR). To capture the impact of COVID-19, additional questions on COVID-19 testing, symptoms and therapy, as well as effect on WM management in those without COVID-19, were included in April 2020.
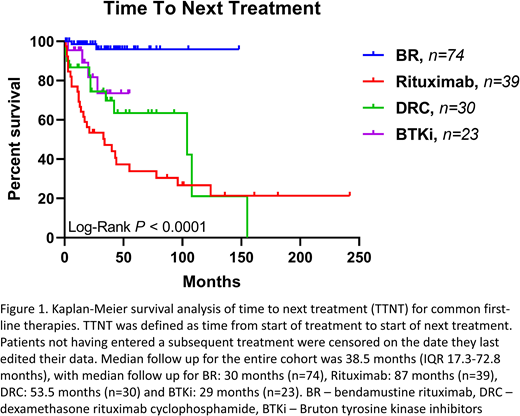
Results: 453 patients from 19 countries have been recruited, predominantly from USA (46%) and Australia (25%), with male predominance (62%). At diagnosis, median age was 61 (range 24-83), median IgM 2620 mg/dL (IQR 1320-3850 mg/dL, n=175) and median hemoglobin 11.4 g/dL (IQR 9.5-12.9 g/dL, n=181). Of the 365 (81%) patients providing symptoms at diagnosis, fatigue/muscle weakness was most common (46%) and 30% were asymptomatic. Using the Impact of Event Scale for symptoms of post-traumatic stress disorder (PTSD) resulting from a cancer diagnosis, the mean score among 387 patients was 5.9 (no stress=0, maximal stress=24), with 39/387 (10%) scoring >13 (PPV 94% for PTSD, Thoresen et al, 2010). This proportion did not increase for scores entered after March 1st, 2020 - 12/123 (10%) - when the COVID-19 pandemic became a global crisis. Marked treatment variation was noted, with 47 different first-line therapeutic combinations documented by 302 patients. Median time from diagnosis to first treatment for USA patients was 48 days (IQR 13-404, n=133) vs Rest of World (ROW) 176 days (IQR 20-885, n=163), (p=0.01). At median follow up of 38.5 months, first-line bendamustine rituximab had superior time to next treatment outcomes compared to other first-line therapies: rituximab monotherapy, dexamethasone-rituximab-cyclophosphamide and Bruton tyrosine kinase inhibitors (BTKi, Figure 1). 51 patients exposed to BTKi had a trend to higher EORTC QLQ-C30 global scales, mean 78.6±17.7, compared to 148 not exposed: mean 73.4±22.6 (p=0.13), despite higher treatment burden: median lines of treatment 2 (IQR 1-4) and 1 (IQR 1-2), respectively (p<0.0001). Paired analysis of global scales entered by patients prior to and after March 1 2020 demonstrated no impact of COVID-19 on quality of life: mean scores 74.4±18.8 and 76.0±17.1, respectively (n=69, p=0.45). Validation of patient-entered data with data-manager-entered data for 31 patients also in LaRDR demonstrated high concordance of >83%. 188/453 (42%) participants responded to the impact of COVID-19 questions; 75/188 (40%) had reduced face-to-face reviews, 4/188 (2%) had delays to starting treatment and 57/188 (30%) documented no impact. Of the 188 respondents, 23 (12%) had COVID-19 testing, with two returning a positive result and neither requiring hospitalization.
Conclusion: WhiMSICAL is a robust, rapidly adaptable, global patient-derived data platform, providing insight into patient symptoms, real-world therapies and PROs. It is a scientific, ethically-approved portal for contributing the patients' voice in this rare lymphoma.
Warden:Janssen Cilag: Other: Personal fees for photoshoot event. Opat:CSL Behring: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AstraZenca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BeiGene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Epizyme: Research Funding. D'Sa:Sanofi: Honoraria; BeiGene: Honoraria, Research Funding; Janssen: Honoraria, Research Funding. Kersten:Takeda: Research Funding; Roche: Consultancy, Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company), Research Funding; Kite/Gilead: Consultancy, Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company); Celgene: Consultancy, Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company), Research Funding; Miltenyi Biotech: Consultancy, Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company); Janssen/Cilag: Consultancy, Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company); BMS: Consultancy, Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company); MSD: Consultancy, Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company); Amgen: Consultancy, Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company); Novartis: Consultancy, Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company). Olszewski:Genentech, Inc.: Research Funding; Adaptive Biotechnologies: Research Funding; TG Therapeutics: Research Funding; Spectrum Pharmaceuticals: Research Funding. Harrington:Calithera Biosciences: Current equity holder in publicly-traded company; Bristol-Myers Squibb: Current equity holder in publicly-traded company; BeiGene: Current equity holder in publicly-traded company; Gilead: Current equity holder in publicly-traded company; Idera Pharmaceuticals: Current equity holder in publicly-traded company; AbbVie: Current equity holder in publicly-traded company. Trotman:Takeda: Research Funding; PCYC: Research Funding; F. Hoffmann-La Roche: Research Funding; Celgene: Research Funding; BeiGene: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.